For the next few blog posts, I thought it would be interesting to cover some of the basic science behind global warming. At one level, it’s a pretty simple concept: The Earth is getting warmer because more greenhouse gases are insulating it. It’s like wearing a winter parka that keeps adding stuffing. But when you think about it a little more, it can get mysterious pretty quickly.
- How come the heat can come in (from the sun), but it gets trapped going out? Does that mean the air is sort of one-way?
- People always say methane is a “powerful” greenhouse gas, a better insulator than carbon dioxide. What makes it so powerful?
- Is water vapor really a greenhouse gas? And if it is, why don’t we hear more about it?
- If we somehow remade the ozone hole, would that help the heat escape? Or is that a dumb question? (For that matter, is ozone a greenhouse gas?)
- Two degrees does not seem like very much. I mean, it could be a rounding error. Can two degrees really make such a big difference?
- What does a “ton” of carbon dioxide mean? A gas-powered car emits around ten “tons” a year. The air can’t hold that much weight, right? So what does it actually mean?
Since a warming planet is our life going forward, and for generations to come, let’s scratch the surface and see if we can answer these questions and more. What are you most interested in? Are there basic questions about how global warming works that are puzzling you? Please share in the comments.

I thought I’d start by going over what happens when the sun shines on our planet. Since I’m not an atmospheric scientist, I'd like to thank Yoichi Shiga, a climate researcher at the Carnegie Institute for Science, for his helpful comments on an earlier version of this blog post.
So, as you know, the sun shines on our planet... As you also know, at least if you have ever bought sunblock, it doesn’t shine just visible light, it also shines ultraviolet (UV) rays. Those are higher energy than visible light and can damage our cells, so we protect our skin with sunscreen. The sun also emits some lower energy rays, called infrared, which is what feels warm. You can see how much of each the sun is shining on us in this graph (source).
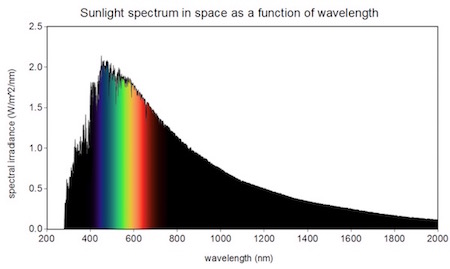
About 40% of the sun’s radiation is visible light, shown between about 400 and 700 nanometers. (1) The shorter and higher-energy ultraviolet rays it emits (10%) are to the left, and the longer, lower-energy infrared (50%) are to the right. (2)
So, that is what’s coming at us. But it doesn’t all get to us. In fact, less than half of the sun’s energy is absorbed by the surface of the Earth.
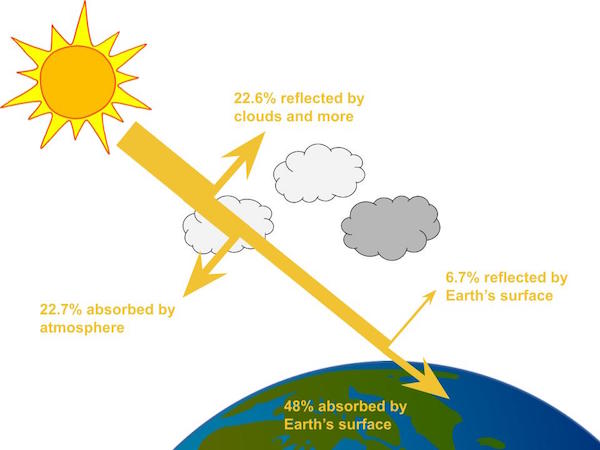
Outgoing energy from the sun, adapted from NASA’s Energy Budget diagram
You can see in the diagram that, to start with, our clouds and atmosphere are reflecting about a quarter of the sun’s energy. You can probably guess that white clouds reflect visible light. But you may not guess that pollution in the atmosphere, such as sulfate aerosols from burning coal and oil, also reflects a good amount of solar energy. So unfortunately, as we clean up the atmosphere by turning down coal plants, we will also be letting through more of the sun’s radiation. Volcanoes are another source of atmospheric pollution. For example, an enormous eruption in the Philippines in 1991 (Mount Pinatubo) ejected 20 million tons of sulfur dioxide and ash up to 12 miles high in our atmosphere. Observations show us that the decreased solar energy reduced global temperatures by about 0.6 C for about 15 months. (3)

Photo of Mount Pinatubo erupting on June 12, 1991, from USGS
Energy that is not reflected by clouds or aerosols may instead be absorbed by the atmosphere. In fact, as shown in the diagram, the atmosphere absorbs another 23% of incoming radiation. What does that mean? Some of the molecules in the atmosphere react to the incoming energy by temporarily changing their structure. Think, for example, of a rubber ball that changes shape when you squeeze it. Similarly, the molecules change shape, or even split or combine, when hit by the sun’s energy. Oxygen absorbs the most dangerous ultraviolet radiation. About 10-20 miles up in the atmosphere, oxygen interacts with the highest energy solar radiation by converting into ozone, keeping the dangerous radiation away from the planet. (4) The lower-energy solar radiation that isn’t strong enough to affect oxygen or ozone molecules passes right through the ozone layer towards Earth.
Finally, the Earth’s surface reflects about 7% of the incoming solar radiation. This reflectiveness is sometimes referred to as the Earth’s “albedo”, and scientists assign albedo values depending on how reflective a surface is. You can imagine that snow, for example, is very reflective (has a high albedo) while dark, wet dirt has a very low albedo. Take a look at the surfaces below, which you might see in a developed area. How would you rank them from lowest to highest albedo? The answer is in footnote 5.

City planners are starting to take this into account, particularly when thinking about urban design, since cities get particularly warm. Ideas including “cool roofs” and “cool streets” are intended to make our surfaces more reflective and cooler.
The undeveloped environment also has natural albedos. Take a look at these two photos of Earth from space. What surfaces do you see, and how would you rank them from lowest to highest albedo? The answer is in footnote 6.
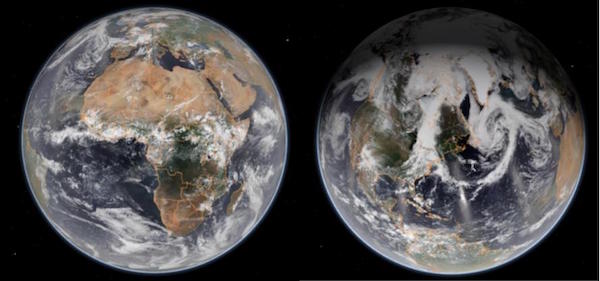
Photos courtesy of NOAA
Scientists are concerned that as the snow and ice melt, converting into darker soils and water, the albedo of the polar areas is dropping and the surface is absorbing more of the sun’s energy, warming faster. This is called a “feedback loop”, in which the warming of the Earth accelerates the warming process itself. In fact, this is one of the main reasons the Arctic is warming twice as fast as average.
There are a number of other factors that influence how much of the sun’s radiation reaches Earth. One of those is sunspots -- more sunspots means more radiation. However, the sun has actually been cooling in recent decades as the Earth is warming. Another factor is the distance of the Earth from the sun, due to variations in the orbit. Every 100,000 years or so, the Earth cycles between closer and farther, which contributes to the coming and going of ice ages. You can find much information on that here. Climate deniers have often pointed to these “natural causes” as explanation for the warming we are seeing. But the rate and scale of today’s warming has deviated well beyond what would be expected in those cases, even if they were aligned with this time in history (which they are not).
So, to sum up, a little more than half of the sun’s energy is reflected or absorbed above the surface of the Earth. That helps to moderate our temperatures. But we are losing some of that reflective capability, for example as snow and ice melt and as sulfate aerosols are reduced. This is accounted for in the climate models that scientists develop, and they are working to better understand and quantify these impacts and evaluate ways to mitigate them. Much of the geo-engineering research work to mitigate global warming revolves around ideas to reduce the amount of solar radiation before it gets to our planet. Given what you learned here, what ideas would you want scientists to think about?
In the next blog post in this series, I’ll talk about what happens to the sun’s energy once it hits Earth and then radiates back out. We’ll learn what happens when the Earth’s surface “absorbs” radiation, what makes some of our gases trap heat, what is an “atmospheric window”, and more…
Current Climate Data (April/May 2019)
Global impacts, US impacts, CO2 metric, Climate dashboard (updated annually)
Notes and References
1. It’s convenient that our eyes evolved to see visible light, given how much the sun emits. In case you are wondering, yes, some animals can see infrared or ultraviolet!
2. Speaking of sunblock, UV A rays are the longest of the ultraviolet (the least energetic), while UV B are somewhat shorter (more energetic). There are even shorter UV C rays, but we don’t hear about them (at least when buying sunblock) because they have so much energy that they get absorbed by the ozone layer. Lucky for us!
3. The stat is from NASA. Earth and Space Science News has an interesting retrospective of the massive Mount Pinatubo eruption and lessons learned.
4. High-energy UV rays are able to split oxygen (O2) molecules into two very unstable oxygen atoms, which then end up forming ozone (O3). Because there is so much oxygen in the atmosphere, almost none of those dangerous rays reach Earth. The ozone itself then reverts back to oxygen, with some help from lower-energy UV rays, releasing a good amount of heat in the process. This is the general process by which the sun’s energy is absorbed by the atmosphere -- energized molecules temporarily transform into “excited” states, then revert, releasing energy that radiates out again.
5. From lowest to highest: fresh asphalt, worn asphalt, grass, dirt, concrete. Fresh asphalt has the lowest albedo, absorbing nearly all of the light energy that comes to it. The albedo value, which is the proportion of solar radiation that is reflected, for fresh asphalt is around 0.04. Something we’ll talk about more in the next post is that “absorb” means that the materials in the asphalt interact with the incoming light, converting much of it to lower-energy infrared (heat) waves. Worn asphalt is about three times as reflective, around 0.12. Next comes grass, at around 0.25. Dirt has a wide range of albedos, with rich wet dirt around 0.1 and light dry dirt around 0.35. The dirt shown in this photo is maybe around 0.3. Finally, traditional concrete has an albedo of around 0.5 when new, dropping as it ages. Albedo values are available at various places online, often shown as a range. I used a few sources, for example here and here.
6. From lowest to highest: oceans, forests, desert sand, then clouds and snow (tied). Our oceans have a very low albedo, around 0.05. That is one reason they are absorbing so much heat. Snow has a very high albedo (0.8 to 0.5, depending on how fresh it is). Thick clouds are generally very reflective (0.7 to 0.8), while thinner clouds have lower albedo. Dry desert sand has a medium albedo (around 0.3), while forests absorb most of the light, with an albedo of 0.1 or 0.2.
7. NASA has a pretty technical but readable overview of how the Earth balances its incoming and outgoing energy.
8. There is a decent (and well illustrated) overview of global warming in Forbes(!)
Comment Guidelines
I hope that your contributions will be an important part of this blog. To keep the discussion productive, please adhere to these guidelines, or your comment may be moderated:
- Avoid disrespectful, disparaging, snide, angry, or ad hominem comments.
- Stay fact-based, and provide references (esp links) as helpful.
- Stay on topic.
- In general, maintain this as a welcoming space for all readers.



