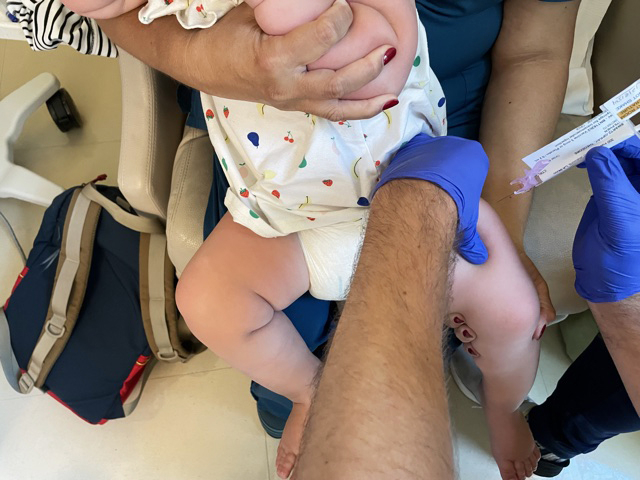
The researcher injected the tiniest needle I've ever seen into my precious toddler's chunky thigh, saying "This is BNT162b2 3 micrograms or placebo in 0.2 mL."
It all started during a Tuesday morning in June. Lying in bed, I scanned my email and found an exciting note: My 1-year-old son, Theo, had been randomly selected to take part in Pfizer's landmark pediatric COVID-19 vaccine trial.
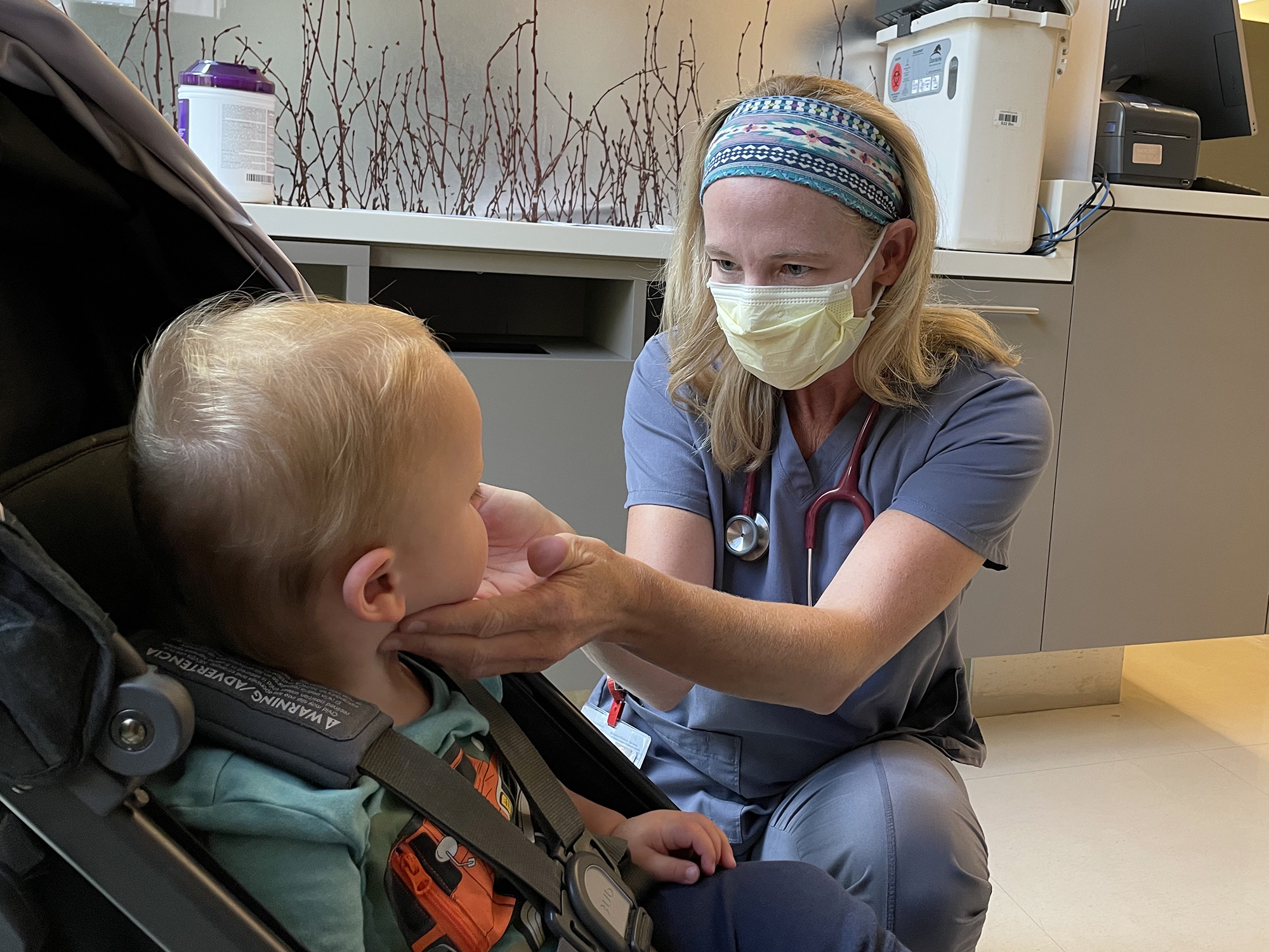
Just 13 months prior, I was in a bed at Stanford Hospital in labor with a face mask due to the pandemic. Now, across the street, my son received his first of two shots in the double-blinded study. He is one of 48 children under 2 who are taking part in the trial being conducted at Stanford University School of Medicine. He is part of a larger cohort of 130 children ranging in age from 6 months to 11 years taking part in Stanford's study. Currently, only children 12 and up are eligible for the COVID-19 vaccine.
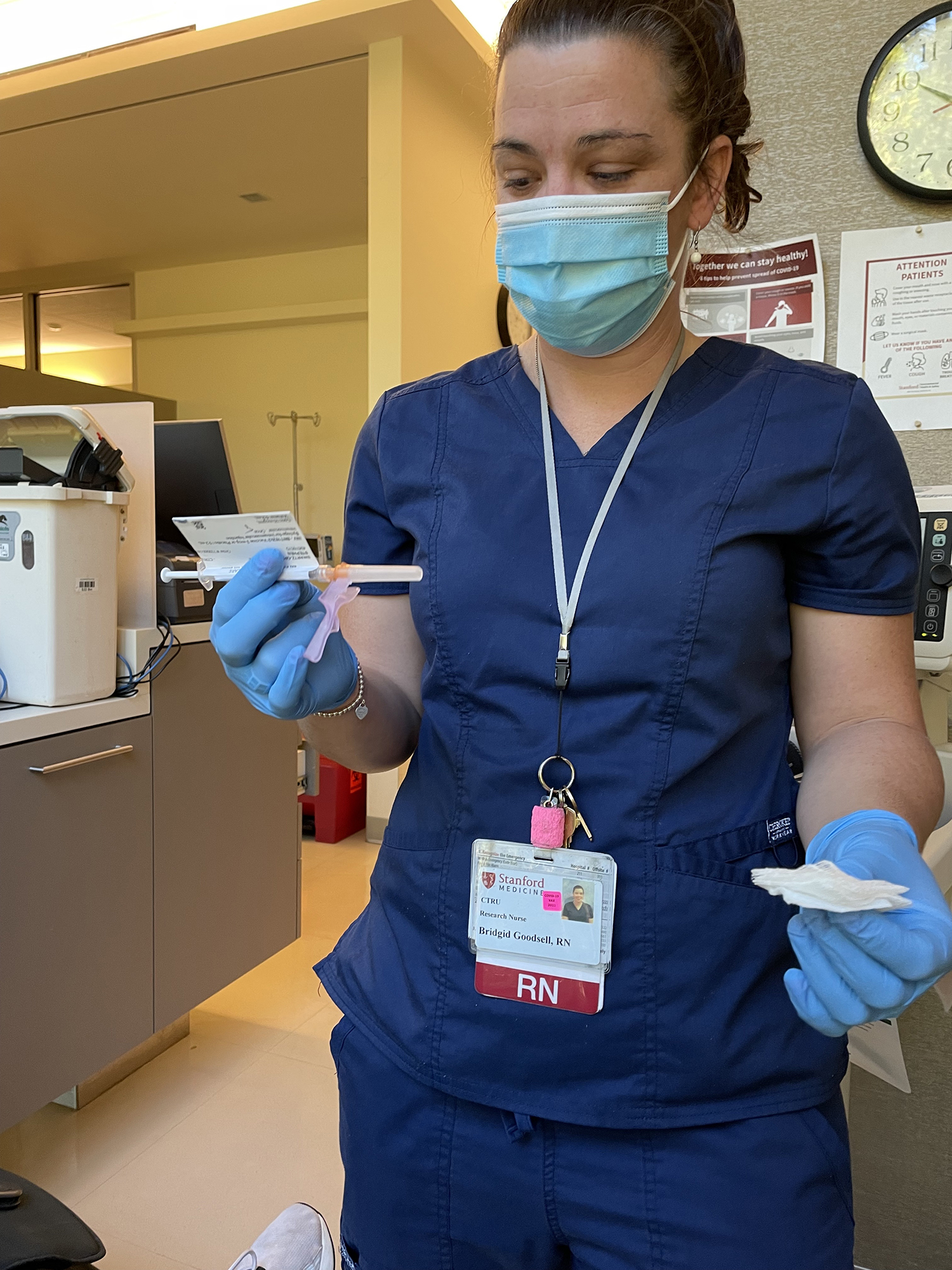
The study has enrolled a little over 4,600 participants, with 101 sites across four countries, said research nurse Jamie Saxena, one of the investigators in the Stanford pediatric study. Pfizer is adding 2,250 children ages 5 to 11 to the study soon, Saxena said. Parents must make a two-year commitment to the trial.
The kids are divided into three age groups: 5- to 11-year-olds; 2- to 5-year-olds; and those 6 months to 2 years old. Reporting from smaller sites means data on the efficacy of the mRNA vaccine on children can be turned over to the FDA for emergency approval faster, she said.
Phase 1 of the study, which began in mid-April at Stanford for the 6 months to 5 years old age group, tested the best vaccine dosage. Theo is in Phase 2/3, which started in mid-May, testing the vaccine's efficacy.
There's a 1 in 3 chance Theo got the placebo instead of the vaccine. The study is unblinded for participants six months after they receive their first shot. At that time, they are offered the vaccine if they didn't receive it already. For us, that will be in January.
If the FDA gives the vaccine emergency approval for the age group sooner, it will be unblinded before that. Media outlets have reported the vaccine could get emergency use authorization for kids ages 5 to 11 as soon as October, after the submission of its trial data to the FDA.
"We are not certain when it will happen for the 6 months to 4-year-old kiddos," Saxena said in an Aug. 16 email.
Getting shots into thighs
I'm not the only parent I know who is eager for children under age 12 to be vaccinated against COVID-19. With the delta variant surging, we'd like to protect our children.
Spots in the study at Stanford University School of Medicine were highly sought after. We applied in April. Over 3,000 families expressed interest in participating in the local study and about 200 were randomly selected, according to a mid-May email sent out to applicants.
I was seven months pregnant when the pandemic took hold in the U.S. I remember checking out at a grocery store, before I stopped going to them entirely in March 2020, with a menagerie of cleaning supplies and being warned that if I was being paranoid about COVID, I would have a paranoid baby. (At the time, we didn't know COVID is airborne and mostly doesn't spread by touching items.)
"How will I protect him?" I cried to my mom on the phone one day, worried about the world I was bringing my tiny human into.
Since the delta variant took hold in the U.S. in early July, a startling number of children across the country are being hospitalized for COVID-19, with an increase of 30% the week of Aug. 9, according to the CDC. Rates are lower in California, where vaccination rates are higher than the rest of the country, masks are required in classrooms and unvaccinated teachers are regularly tested, according to the Los Angeles Times.
The risk from participating in the vaccine trial is much lower than that of being hospitalized with COVID-19, Saxena told me. "Adults have been receiving this vaccine for a year and adolescents have been as well," she said. "Through all of the previous people who have received the vaccine, it's shown that it's safe and it's shown that it's effective."
Theo's pediatrician, Dr. Jeffrey Tan, told me more parents are asking him about when the vaccine might be approved for their young children. Parents are also wondering what activities they can resume if they're vaccinated but their children aren't.
"It's a tough question to answer," he said. "The under 12 (kids) really are still at risk. In a crowded setting, a park or an airplane, there's no way to tell them there's no risk."
He said he generally points parents to CDC guidelines.
Not every parent is eager to have their children vaccinated against COVID. Tan said some parents are always a little more hesitant about vaccines in general.
"It's understandable because it's a new vaccine," he said. "The CDC recommends it because the benefit of the vaccine outweighs any risk that has been detected so far. Kids get sick from COVID; they do get hospitalized. ... We're not out of the woods yet."
A hiccup in the trial
June 25 was marked on my calendar for Theo's first shot. I got an early morning phone call that day saying his appointment was canceled. The concern? Hundreds of reports of heart inflammation in young adults.
Pfizer put the study on hold while clinics rewrote the consent form with a warning that myocarditis, inflammation of the heart muscle, and pericarditis, inflammation of the lining outside the heart, have occurred with some younger people who've received shots.
In both cases, the body's immune system is causing inflammation in response to an infection or some other trigger. Symptoms can include chest pain, shortness of breath or palpitations, according to the CDC.
The severity of cases of myocarditis and pericarditis can vary. For the cases reported after mRNA COVID-19 vaccination, most who sought medical care responded well to medications and rest. There also tends to be a spike in illnesses that cause these same inflammatory responses in the summer, so researchers are unclear if the vaccine is truly the cause, clinicians told me. The symptoms of both also tend to resolve on their own without medical intervention.
The delay was disappointing, but I was glad they were being thorough. I talked to Theo's pediatrician and the researchers. Because Theo is very healthy, my husband and I felt comfortable continuing in the study. To my mind, helping bring the protection of a vaccine against COVID-19 to children outweighed the risk of a rare complication.
And it's not just about protecting the estimated 48 million Americans under age 12, but everyone else who is at risk because of the growing number of breakthrough infections from the highly contagious delta variant.
Saran Wrap and tiny needles
I pushed Theo in his stroller into the vaccine clinic on Welch Road for his first shot in the trial on a Tuesday morning in July, juggling all the things that accompany toddlers: snacks, toys and milk. The initial visit was set to last between two and three hours but ended up taking about three and half hours.
I reviewed the consent forms with a researcher, then they measured Theo's height, weight and blood pressure. They loaded $100 on a debit card as compensation for taking part in the study and said they'd add $5 for each electronic diary entry on his symptoms we completed.
They also swabbed his nose to take a COVID test. Lots of kicking and trying to grab the swab ensued.
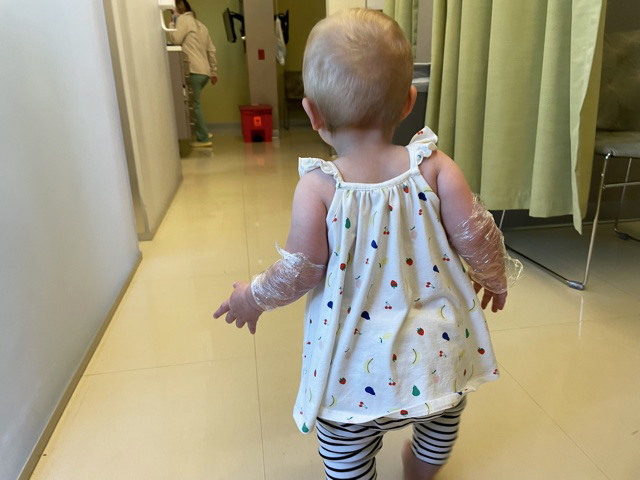
It was difficult for me to watch the blood draw to check for COVID antibodies. He wore numbing Emla cream covered with Saran Wrap — so he couldn't rub it off — for about 30 minutes beforehand. His tiny arm was bruised for about a week after, but it didn't look awful.
The hardest part of the appointment was waiting for the shot to arrive. Theo grew bored and wanted to wander the halls, but the vaccine was delayed from the pharmacy and it has to thaw once it arrives. The needle was so small! Children under 2 in the trial receive 3 micrograms of the vaccine, while 5- to 12-year-olds receive 10 micrograms. The adult dose is 30 micrograms.
After the shot we went out to the playground while we waited 30 minutes to see if he had any adverse reactions. He didn't have any then, or for the following week, which lines up with what clinicians are seeing in other children under 2 in the study: a little extra tiredness and irritability, but no fevers, Saxena said.
For the next week, every evening we logged his temperature, indicated if he had any injection site redness, rash, tenderness, fatigue and other symptoms in the "TrialMax" app. Every week, we update whether he's shown any symptoms of COVID-19.
They also sent us home with an at-home COVID test that I can use if he has any virus symptoms.
The second visit
The next visit in early August was thankfully much shorter — an hour total.
It was similar to the first one, but without the blood draw. Again, we waited 30 minutes to see if he had any symptoms from the shot and then left.
He had a very slight fever of about 100 degrees after the second shot, but nothing more.
In September we'll have a phone check-in about how Theo is doing. He will also have another antibodies test.
'Making history'
I'm excited to tell Theo when he's older about his participation in a study that hopefully helps end this devastating pandemic. It's one way we can show that we care for others.
Saxena told me it's an "incredible honor" for her to be part of Pzifer's study.
"As a clinician, you always read research articles and I always thought of them as being the guiding principles for medicine," she said. "People who do clinical trials are the experts in the field. To be literally leading a trial the entire U.S. has their eyes on, to me feels like I'm part of one of the most important things going on in the U.S. right now," adding that it would probably be the apex of her career.
"I still have no idea how I got so lucky to be part of this," she said. "The kids that are in the study are really making history; without their participation, they would not have data to bring to the FDA. The kids are really moving medicine forward and helping to control COVID in the pediatric population."
As parents we make endless choices for our children — use cloth diapers or disposable? Formula or breast milk? Sleep training or not? We make the best decisions we can with the information we have on hand, evaluating risk along with our children's safety and happiness. You come to realize as a parent there's only so much you can control. I hope Theo received the vaccine, but we'll just have to wait and see.
For more on the trial, go here.
If you have questions about the COVID-19 vaccines, visit our FAQ page here.
Read the follow-up story, Three vaccine shots later: Being part of Pfizer's trial means my son is one of the few toddlers protected against COVID-19.






Comments
Registered user
Monta Loma
on Aug 27, 2021 at 4:48 pm
Registered user
on Aug 27, 2021 at 4:48 pm
[Post removed due to disinformation]
Registered user
Another Mountain View Neighborhood
on Aug 28, 2021 at 10:04 am
Registered user
on Aug 28, 2021 at 10:04 am
Thank you for the in-depth article on this process and looking forward to the vaccine's approval for our youngest ones.